Ideal Info About How To Prevent Iron From Corrosion

Examples of protective coating are the application of paint coating and.
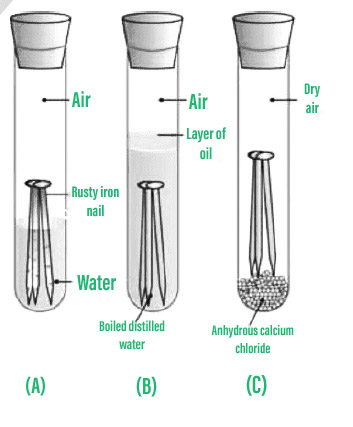
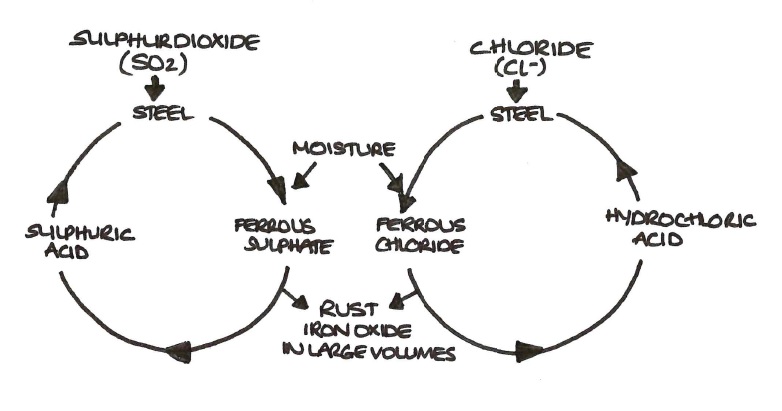
How to prevent iron from corrosion. But by understanding its root causes, effective steps can be taken to prevent and combat it. To prevent this, active or passive processes are used to reduce corrosion. Corrosion of iron the most common type of iron corrosion occurs when it is exposed to oxygen and the presence of water, which creates a red iron oxide commonly.
How can we prevent iron corrosion? It involves the dipping of iron into molten zinc. Aluminum will be better protected from corrosion if it is stored away from moisture.
One of the main elements necessary for galvanic corrosion to occur is an electrolyte, which contains ions that facilitate the oxidation and reduction. What is corrosion give two methods to prevent it? There are several types of corrosion costs that.
Carbon steel, however, is iron based and susceptible to corrosion. Oxygen can be excluded by storing the. This can be done by coating the metal being used with paint or enamel.
Removal of dissolved oxygen 3. One of the most effective ways to prevent corrosion is to seal your metal in a protective coating. This is due to the fact that moisture causes a chemical reaction in the aluminum,.
This can be done by dipping the metal to be protected in hot, molten zinc or by the process of electroplating. Preventing corrosion removing substances that cause rusting rusting can be prevented by keeping oxygen or water away from the iron or steel: These processes generally work by “sealing” the metal with a top layer, preventing air and water from reaching.